# Introduction:
Sulphur Dye is mainly insoluble in water. It’s a very familiar di-cellulosic fiber and a important part of Sulphur linkage, Sulphur dye Chromophore (it’s a color bearing group). It called Sulphur dye because of Sulfide (-s-), di-sulfide (-s-s-) and polysulphide (-sn-) these bond. It contains disulfide linkage with two or more chromophore group. Sulphur dye is use to produce rich black and highly black color. Another dye name vat dye which is very similar to the Sulphur dye.
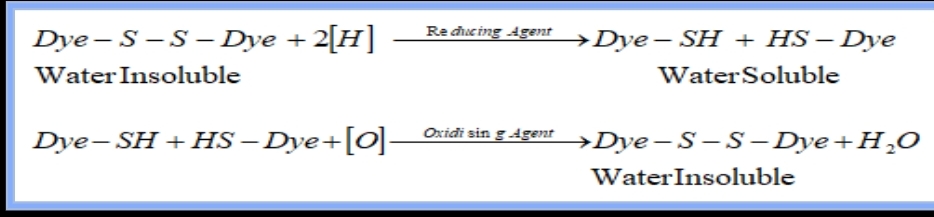
## The reaction for producing pure Sulphur dye given below
## History of Sulphur Dyes:
- In 1873, sawdust was heated with sulphur and caustic soda to create the first Sulphur dye. Cotton was able to absorb this material from alkaline liquid, and when it was oxidized with potassium dichromate, a brown colour with good wet-fastness was created.
- The true inventor of sulfur dyes, Vidal, created the Vidal blacks in 1893 by combining sodium Sulfide, Sulphur, and paraphenylenediamine, also known as para-aminophenol. This specific color, however, was never commercially successful. By heating 2:4-dinitro-4′ hydroxy diphenylamine with sodium polysulfide in 1897, Kalischer created Immediate Black FF.
- Because they need a reduction agent and a somewhat alkaline environment to bond the color to the fabric, sulphur dyes are special. This technique is called “sulphur dyeing.” Deep and rich colors can be produced with sulphur dyes, which are renowned for their fading resilience.
- One of the key developments in the history of sulfur dyes was the introduction of the “sulfur black” dye in the 1920s.
- Over the years, Sulphur dyes became more efficient and eco-friendly, because of the advancements in the production.
## Properties of Sulphur Dye:
- It contains hydroxy amino or nitro group.
- It is a water insoluble dye
- It needs reducing agent to solubilize in water
- It works in alkaline condition
- It needs oxidation before producing the final color or being stable.
- It is caused for the salt exhaustion
- We need the temperature 80-90°C to dye a material with this chemical
- It gives average color fastness properties, which can improve by following proper steps.
- It is a suitable dye for cotton and viscose
- It is cheaper and easier to manufacture than other dyes
- No chance of staining or bleeding. It means it doesn’t place from the fabric surface or in the time of washing it doesn’t spread over the other fabric.
- They are exclusively amorphous, few of them show crystallinity.
- Heavy and durable shades are produce by this dye.
##Types of Sulphur dyes:
- CI Sulphur dyes
- CI leuco Sulphur dyes
- CI solubilised Sulphur dyes
- CI condensed Sulphur dyes
## Exhaustion of Sulphur dyes:
- Exhaustion of dyeing means when a Textile material or fabric dived into the water than amount of dye absorb, dyeing rate, dye uptake rate, the time of dyeing, amount of dye remain in the dye batch.
- When we engross a fabric in the dye liquor than the rate of absorb dye is high in the initial stage. After a certain time it reach in it’s equilibrium stage. In this stage, no dye migrate on the fabric surface it is happens for exhaustion of dyes
- In the second stage, we use glauber salt to encourage the exhaustion rate to increase the amount of dyeing and decrease in the amount of dye liquor to produce a good color on the surface of that fabric.
Mechanism of Sulphur dyeing: The molecules of the sulfur dyes have sulfur linkage in them. They show substantivity toward cellulose and are insoluble in water, but they can be reduced to become soluble in water by treating with reducing chemicals. The reaction of dye is followed:
Dye-s-s-dye-Na2s + Na2CO3 == Dye-S-H +H-S-Dye + [O]== Dye-S-S +H2O
(Insoluble Stage) (Solubilizing Group) (Soluble Stage) (oxidation) (Again insoluble)
We know that Sulphur dye doesn’t have a solubilizing group, it’s insoluble. When we use a reducing agent (Sodium Sulphide, sodium hydrosulphide), then we achieve a solubilizing group in this dye. This group adds with the dye by breaking di-sulphide linkage. Then we get a soluble stage of sulphur dye than the fabric needs to dye with sulphur dye for a certain amount of time. Then addition of will increase the exhaustion rate and help to produce final color. Once, the shade matches the required hue, then the oxidation (Hydrogen peroxide, Sodium Bromide) process needs to run in order to make the dye insoluble again. Otherwise, color fastness properties won’t develop.
To get rid from this type of problem, Commercially leuco form(soluble form) is produced by many industries. An iron vessel isn’t used in sulphur dye because iron reacts with sulphur.
Classification of Sulphur dye:
- CI sulphur dye: It is a basic sulphur dye, which is water insoluble. To dye the material with this type of dye, we need to soluble it then dye, then oxidize to gain insoluble form again.
- CI leuco Sulphur dye: It is a ready made soluble sulphur dye. It remains in soluble form because of adding reducing agents from the first. It doesn’t contain a solubilized group.
- CI solubilized sulphur dye: It is a naturally soluble sulphur dye. It means the dye contain a solubilized group from its chemical construction.
- CI Condensed sulphur dye: This dye is in condensed form.
## Commercial forms of sulphur dye: Sulphur dye can be found in powder, grains, disperse powder and disperse paste form.
Application method: In case, sulphur dye is water insoluble. So, we need to use a reducing agent and oxidizing agent to dye a material with this dye. The method of reduction and oxidation is followed:
- Reduction method: The reducing agents used in sulphur dye are Na- Sulphide, Na- Hydrosulphide, Pseudo-thio-hydanton. For dissolving sulphur dye reducing agents are used. For example we need a 9 gm reducing agent to dissolve 9 gm sulphur dye. Mainly Na-Sulphide is used to dissolve sulphur dye. Na-hydrosulphide is also used but it can cause poor wash fastness sometimes and also decrease the lifetime of the vessel.
- Oxidation method: After dyeing, it is compulsory to make the dye in insoluble form. So, we use the oxidation process to make it insoluble. The common oxidizing agents are Hydrogen Peroxide, Na- Peroxide, K- dichromate. This methods play an important role in the development of the color shades and optimum fastness properties. Utilizing various oxidizing agents may result in the following outcomes:
- A brighter shade appears while perborate or percartborate is used in the presence of acetic acid.
- Dichromate treatment without rinsing lessens color loss but results in a dull color.
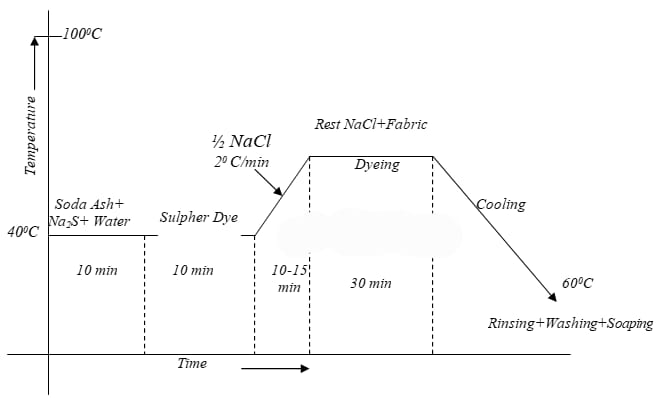
Dyeing of cellulosics with sulphur dye: The sequence of dyeing with sulphur dyes are-
Goods preparation ➤ Dye solution preparation ➤ Dyeing ➤ Oxidation ➤ After treatment ➤ dyed goods
Typical Recipe:
The Typical recipe for dyeing is as below:-
- Sulphur Dye : 10% (On the weight of the fabric)
- Na2S (Reducing Agent) : 1.5% (On the weight of the Dye)
- Salt (NaCl) : 8 g/l
- Soda Ash (NaCO3) : 7 g/l
- Temperature : 1000C
- Time : 90 min.
- Material: Liquor : 1:20
Conclusion: We already know that sulphur dye contains a di-sulphide linkage. For this linkage, it can produce sulphur dioxide in the environment. Which is mainly responsible for Acid rain, which causes deforestation. This Acid rain has a great impact on Terrestrial and aquatic life. So, we need to make this dye water soluble before releasing it to the environment. Sulfur dyes have played a significant role in the history of textile dyeing, and they continue to be used in various industries today.
Abrar Sadman Arpon
Department of Textile Engineering
Jashore University of Science and Technology


